All atoms of hydrogen are alike in that each atom has the same. Proton 3Which among the following.

Atom Test 8th Grade Science Multiple Choice Test Atom
They contain 1 and 3 protons respectively.
. An atom has 3 protons 4 neutrons and 3 electrons. View the full answer. According to atomic number.
A Structurally variant atoms which always have a mass number of 1. The molecule is in the solid form at room temperature. Chemistry questions and answers.
Neutrons carry a positive charge. 1 point Which of the following best describes the absorption of a photon by an elektron in an atom. 1Which of the following best describes an atom.
A small walled spheres that contain electrons. The bond in the molecule is polar covalent. Which of these phrases best describes an atom.
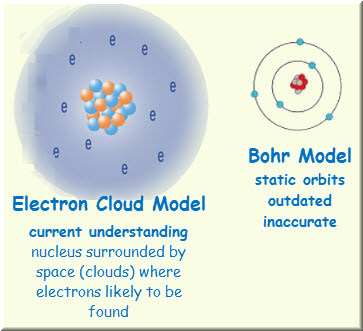
Which one of the following best describes the simplest unit of MgCl2. B Structurally variant atoms which have the same number of neutrons and protons but differ in the number of electrons they contain. SPS1a DOK 1 O protons and electrons grouped together in a random pattern O protons and electrons grouped together in an alternating pattern a core of protons and neutrons surrounded by electrons O a core of electrons and neutrons surrounded by protons.
By the number of. 1 Mg atom and 2 Cl atoms e. Atom is an smallest unit of an Element.
An atom has 3 protons 4 neutrons and 3 electrons. Neutrons move around a nucleus. A12 protons 12 neutrons and 12 electrons B11 protons 11 neutrons and.
2Which subatomic particles is electrically neutral. Which of the following best describes an atom. Atom is the basic unit of matter.
85A An atom has 25 protons 30 neutrons and 25 electrons. 16_ 16 Which of the following best describes how an atom with atomic number 12 would behave in terms of forming bonds with. Which of the following best describes an isotope.
We review their content and use your feedback to keep the quality high. Atom is made up of. Get the answers you need now.
Which of the following best describes the relationship between the atoms described below. Which of the following best describes an orbital. Nucleus with 7 protons and 7 neutrons surrounded by 7 electrons Both atoms have the same atomic number but different atomic mass numbers.
Is it C 2 A covalent bond is. 1 MgCl2 molecule c. 100 1 rating Mg is the atom whose atomic number is 12.
If the green sphere represents a chlorine atom which of the following best describes the picture below. The molecule can separate in the water to produce 2 Clions. D space which may contain electrons protons andor neutrons.
20 Questions Show answers. 5 When an atom emits light an electron in it moves from a higher to a lower energy level. 1 Mg atom and 1 Cl2 molecule b.
C the space in an atom where an electron is most likely to be found. 1 See answer Protons carry a negative charge. Atom 1 Atom 2 1н 1 Зн They each contain only 1 neutron.
Which one of the following statements best describes electronegativity in atoms. B a single space within an atom that contains all electrons of that atom. Use the periodic table to determine which atom would have similar chemical properties to this atom.
Which of the following best describes the particles in an atom. Nucleus with 7 protons and 8 neutrons surrounded by 7 electrons Atom 2. 4 Which statement best describes the process during which an electron in an atom emits light.
Of the following the group that contains elements that are the most reactive is the. 85A Which of these best describes one of the subatomic particles that could be found at location X in the model of an atom shown above. C Electronegativity is the energy lost when an atom gains an electron.
An isotope is an atom of an element that varies in mass number due to variation in the number of. 85A Which of the following best describes an electron. Which of the following best describes an atom.
An electron moves to a lower energy level and releases a photon O an electron absorbs the energy from all photons of light and the electron will move further away from the nucleus O electrons do not absorb energy in the form of photons o an electron absorbs the. They can do so by achie. B Electronegativity is the attraction an elements nucleus has for the electrons in a chemical bond.
3 How is light emitted from an atom quizlet. Use the periodic table to determine which atom would have similar chemical properties to this atom. Which statement about subatomic particles is NOT true.
What is the charge of the atoms nucleus. Andreadacosta69 andreadacosta69 11182020 Chemistry High School answered Which of the following best describes the particles in an atom. Atom 1 Atom 2 1н 1 Зн They each contain only 1 neutron.
One chlorine atom can not exist naturally. Any atom in the periodic table wants to be stable. 1 Which Of The Following Best Explains What Is Happening When An Atom Emits Light.
Which of the following statement best describes an 1Which of the following statement best describes an atom. How many electrons are in each energy. A Electronegativity is what happens when an atom gains an electron to become an anion.
2 What is happening when an atom emits light. A model of an atom shows eight electrons in rings that represent different energy levels. 1 positive Mg ion and 2 negative Cl ions d.
In the modern periodic table elements are arranged. The attraction that one atom has for another atom.

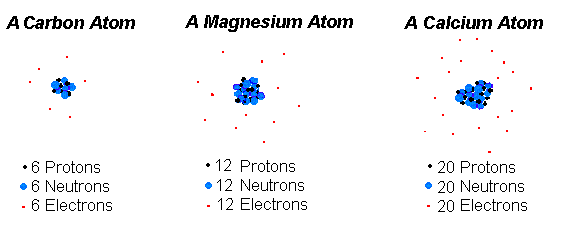
Labeled Parts Of An Atom Diagram Atom Diagram Atom Worksheets
0 Comments